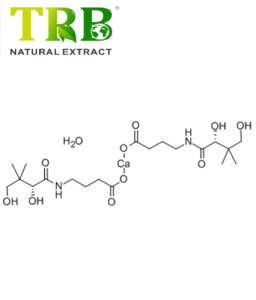
Product Name:1-(methylsulfonyl)spiro[indoline-3,OtherName:1,2-dihydro-1-(methylsulfonyl)-Spiro[3H-indole-3,4'-piperidine];Spiro[3H-indole-3,4'-piperidine],1,2-dihydro-1-(methylsulfonyl)-;1-methylsulfonylspiro[2H-indole-3,4'-piperidine]
CAS NO.:178261-41-1
Assay: 98.0%
Colour: off-white powder
Packing:25kg/DRUMS
1-(Methylsulfonyl)spiro[indoline-3,4'-piperidine]: A Potent c-Met/ALK Dual Inhibitor for Oncology Research
Product Overview
1-(Methylsulfonyl)spiro[indoline-3,4'-piperidine] (CAS: 178261-41-1) is a spirocyclic compound with a molecular formula of C₁₃H₁₉ClN₂O₂S and a molecular weight of 302.83. This high-purity (>95%) small molecule is recognized for its privileged scaffold in kinase inhibitor design, particularly targeting c-Met (hepatocyte growth factor receptor) and ALK (anaplastic lymphoma kinase), two critical oncogenic drivers in cancers .
Pharmacological Activity & Applications
1. Dual c-Met/ALK Inhibition
The compound’s spiro[indoline-3,4′-piperidine] core enables potent and selective inhibition of c-Met and ALK kinases. Preclinical studies demonstrate:
- Single-digit nM biochemical potency against c-Met and ALK .
- In vivo efficacy in suppressing tumor growth (>50% inhibition) in gastric carcinoma xenograft models .
- Oral bioavailability and favorable pharmacokinetics, making it suitable for therapeutic development .
2. Mechanism of Action (MoA)
The methylsulfonyl group enhances solubility and binding affinity, while the spiro structure allows solvent-exposed interactions with kinase domains, minimizing off-target effects . This selectivity is validated by 97-kinase panel screening, where the compound showed minimal cross-reactivity .
3. Therapeutic Potential
- Targets cancers with dysregulated c-Met/ALK signaling, including non-small cell lung cancer (NSCLC), gastric carcinoma, and glioblastoma .
- Acts as a chemically versatile intermediate for optimizing analogs with improved pharmacokinetic profiles .Synthesis & Quality Assurance
1. Efficient Synthetic Routes
- Four-component reactions involving isatin, methyl propiolate, and arylamines yield functionalized spiro[indoline-3,4'-piperidine] derivatives with high regioselectivity .
- Suzuki coupling and Mitsunobu reactions enable modular functionalization of the core structure for diverse analogs .
2. Quality Control
Supplied by reliable manufacturers (e.g., Bellingham & Stanley, Matrix Scientific), the product undergoes rigorous HPLC and NMR verification to ensure ≥95% purity .
Research Support & Citations
- Key References:Molecular Docking: Structural studies confirm interactions with kinase hinge regions, supporting rational drug design .
- Li et al. (2013) identified spiro[indoline-3,4′-piperidine]-2-ones as selective c-Met/ALK inhibitors .
- Zhang et al. (2013) developed one-pot syntheses for spiro compounds, highlighting scalable methods .
Conclusion
1-(Methylsulfonyl)spiro[indoline-3,4'-piperidine] is a promising candidate for oncology research, combining high selectivity, oral efficacy, and synthetic accessibility. Its applications span from lead compound optimization to mechanistic studies in kinase-driven cancers.
Supplier Information
- CAS: 178261-41-1 | Purity: >95%
- Availability: Stocked by global suppliers (e.g., ChemNet, Bellingham & Stanley) .
- Contact: info@trbextract.com
Keywords: c-Met inhibitor, ALK inhibitor, spiro[indoline-3,4′-piperidine], kinase inhibitor synthesis, oncology research compounds.
![1-(methylsulfonyl)spiro[indoline-3,4'-piperidine] Featured Image](https://www.trbextract.com/uploads/178261-41-1.png)
![1-(methylsulfonyl)spiro[indoline-3,4'-piperidine]](https://www.trbextract.com/uploads/178261-41-1-287x300.png)