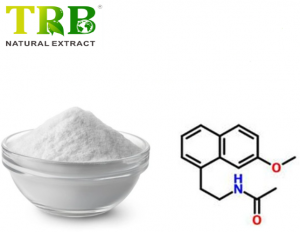
Product Name:Agomelatine
Other Name:N-[2-(7-Methoxy-1-naphthyl)ethyl]acetamide;N-[2-(7methoxynaphthalen-1-yl)ethyl]acetamide
CAS No:138112-76-2
Specifications: 99.0%
Colour:White fine powder with characteristic odor and taste
GMO Status:GMO Free
Packing: in 25kgs fiber drums
Storage:Keep container unopened in cool, dry place,Keep away from strong light
Shelf Life:24 months from date of production
Agomelatine Product Description
1. Product Overview
Agomelatine is a novel antidepressant with a unique dual mechanism of action: it acts as a melatonin receptor agonist (MT1/MT2) and a 5-HT2C receptor antagonist**. Its chemical name is N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide, with the molecular formula C₁₅H₁₇NO₂ and CAS number 138112-76-2 . Available in powder or tablet form (25 mg per tablet), it is highly pure (≥98% by HPLC) and soluble in organic solvents like DMSO and ethanol.
2. Indications
Agomelatine is indicated for the treatment of major depressive disorder (MDD) in adults, particularly for patients intolerant to conventional antidepressants or with comorbid anxiety or sleep disturbances . Clinical trials demonstrate efficacy in improving depressive symptoms, circadian rhythm regulation, and daytime functioning.
3. Mechanism of Action
- MT1/MT2 Agonism: Restores circadian rhythms by mimicking melatonin, enhancing sleep quality and daytime alertness**.
- 5-HT2C Antagonism: Increases dopamine and norepinephrine release in the prefrontal cortex, alleviating depressive symptoms.
This dual action differentiates Agomelatine from SSRIs/SNRIs, offering a favorable tolerability profile.
4. Dosage and Administration
- Recommended Dose: 25–50 mg once daily at bedtime**.
- Pharmacokinetics: Rapid absorption (peak plasma concentration within 2 hours), metabolized via CYP1A2, and excreted renally**.
5. Clinical Efficacy
- Superiority in Circadian Regulation: Agomelatine significantly improves sleep latency and efficiency compared to sertraline, with effects observed as early as Week 1**.
- Long-term Benefits: Maintains remission in MDD over 1 year, with low relapse rates and minimal discontinuation due to adverse effects**.
- Anxiety and Functionality: Reduces anxiety symptoms and enhances work, social, and family functioning**.
6. Safety and Tolerability
- Common Side Effects: Headache, nasopharyngitis, and mild gastrointestinal disturbances**.
- Safety Profile: No significant weight gain, sexual dysfunction, or withdrawal symptoms, aligning with its receptor selectivity**.
- Environmental Caution: Classified as aquatic toxicant (WGK 3); avoid direct environmental exposure**.
7. Storage and Handling
- Storage: Store at 2–8°C (powder) or room temperature (tablets in original packaging)**.
- Stability: Stable for ≥2 years when protected from light and moisture**.
8. Regulatory Status
- Approved in the EU, Australia, and other markets under brand names like Valdoxan®**.
- Listed in WHO International Nonproprietary Names (INN) and FDA Preferred Term databases**.
9. Research Applications
Agomelatine is also used in preclinical studies for neuroprotection, tau protein phosphorylation, and calcium signaling in neurons . Reference standards (e.g., impurities, metabolites) are available for pharmaceutical development and quality control.
Keywords : Agmelatine, MT1/MT2 agonist, 5-HT2C antagonist, major depressive disorder, circadian rhythm, Valdoxan, antidepressant efficacy, serotonin receptor, melatonin analog.