We enjoy an extremely good status among our prospects for our great merchandise top quality, competitive price and the ideal service for Andrographis Paniculata Leaf Extract Andrographolide, Welcome your browsing and any your inquires,sincerely hope we will have chance to cooperate along with you and we can easily build up lengthy well small business romantic relationship with you.
We enjoy an extremely good status among our prospects for our great merchandise top quality, competitive price and the ideal service for China Herbs and Plant Extract, At present our sales network is growing continually, improving service quality to meet customer’s demand. If you are interested in any products and solutions , be sure to contact us at anytime. We are looking forward to forming successful business relationships with you in near future.
Andrographis Paniculata is an herb commonly used in China, India, and other countries in subtropical and Southeast Asia. Both the fresh and dried leaves, as well as the fresh juice of the whole plant, have been used in a variety of cultures.
The most common therapeutic potential of Andrographis Paniculata is its liver protective property. Some clinical studies demonstrates its liver protective effects by enhancing activity of antioxidant enzymes along with the level of glutathione and decreasing the activity of lipid peroxidase which leads to generation of free radicals damaging the liver cells thus exerting a Hepatoprotective activity.
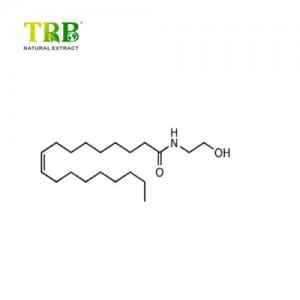
Product Name:Andrographis Paniculata Extract
Latin Name:Andrographis Paniculata (Burm.f.) Nees
CAS No.:5508-58-7
Plant Part Used:Aerial Part
Assay: Andrographolide 10.0%-98.0% by HPLC
Colour: light white powder with characteristic odor and taste
GMO Status:GMO Free
Packing: in 25kgs fiber drums
Storage:Keep container unopened in cool, dry place,Keep away from strong light
Shelf Life:24 months from date of production
Function:
-Andrographolide can protect skin from pimples.
-Andrographolide can be used as blood sugar reducer.
-Andrographolide has effect on fighting bacterial activity.
-Andrographolide can kill intestinal worms and supports ntestines.
-Andrographolide can reduce swelling and cuts down exudation from capillaries.
-Andrographolide also can reduce diarrhea and symptoms arising from bacterial infections.
-Andrographolide has the function of promoting mucus discharge from the respiratory system.
-Andrographolide can help reduce symptom severity in people with common colds. Andrographolide also said to decrease fertility.
Application:
-Applied in veterinary field , it is made into pulvis to treat acute bacillary dysentery , gastro-enteritis and pneumonia of poultry and livestock.
-Applied in pharmaceutical field, it is usually made into tablets, soft capsule, injection, etc. To treat acute bacillary dysentery, gastroenteritis ,cat fever, amygdalitis, faucitis, pneumonia, phthisis and so on.
TECHNICAL DATA SHEET
| Item | Specification | Method | Result |
| Identification | Positive Reaction | N/A | Complies |
| Extract Solvents | Water/Ethanol | N/A | Complies |
| Particle size | 100% pass 80 mesh | USP/Ph.Eur | Complies |
| Bulk density | 0.45 ~ 0.65 g/ml | USP/Ph.Eur | Complies |
| Loss on drying | ≤5.0% | USP/Ph.Eur | Complies |
| Sulphated Ash | ≤5.0% | USP/Ph.Eur | Complies |
| Lead(Pb) | ≤1.0mg/kg | USP/Ph.Eur | Complies |
| Arsenic(As) | ≤1.0mg/kg | USP/Ph.Eur | Complies |
| Cadmium(Cd) | ≤1.0mg/kg | USP/Ph.Eur | Complies |
| Solvents Residue | USP/Ph.Eur | USP/Ph.Eur | Complies |
| Pesticides Residue | Negative | USP/Ph.Eur | Complies |
| Microbiological Control | |||
| otal bacterial count | ≤1000cfu/g | USP/Ph.Eur | Complies |
| Yeast & mold | ≤100cfu/g | USP/Ph.Eur | Complies |
| Salmonella | Negative | USP/Ph.Eur | Complies |
| E.Coli | Negative | USP/Ph.Eur | Complies |
|
More information of TRB |
||
| Regulation certification | ||
| U.S.FDA,CEP,KOSHER HALAL GMP ISO Certificates | ||
| Reliable Quality | ||
| Nearly 20 years,export 40 countries and regions,more than 2000 batches produced by TRB have no any quality problems,unique purification process,impurity and purity control meet USP,EP and CP | ||
| Comprehensive Quality System | ||
|
|
▲Quality Assurance System |
√ |
| ▲ Document control |
√ |
|
| ▲ Validation System |
√ |
|
| ▲ Training System |
√ |
|
| ▲ Internal Audit Protocol |
√ |
|
| ▲ Suppler Audit System |
√ |
|
| ▲ Equipment Facilities System |
√ |
|
| ▲ Material Control System |
√ |
|
| ▲ Production Control System |
√ |
|
| ▲ Packaging Labeling System |
√ |
|
| ▲ Laboratory Control System |
√ |
|
| ▲ Verification Validation System |
√ |
|
| ▲ Regulatory Affairs System |
√ |
|
| Control Whole Sources and Processes | ||
| Strictly controlled all raw material,accessories and packaging materials.Preferred raw materials and accessories and packaging materials supplier with U.S. DMF number.
Several raw material suppliers as supply assurance. |
||
| Strong Cooperative Institutions to support | ||
| Institute of botany/Institution of microbiology/Academy of Science and Technology/University | ||