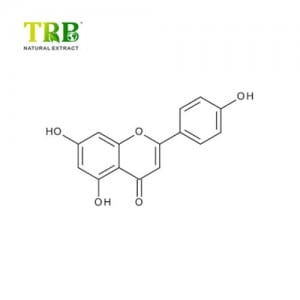
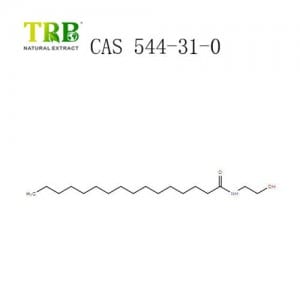
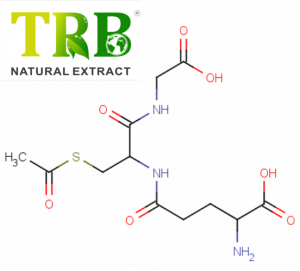
Product Name:Bee Venom
CAS No:20449-79-0
Assay:Apitoxin≧99.0% by HPLC
Colour:LIght yellow with characteristic odor and taste
GMO Status:GMO Free
Solubility:100% soluble in water
Storage:Keep container unopened in cool, dry place,Keep away from strong light
Shelf Life:36 months from date of production
Natural Bee Venom: Therapeutic Benefits and Skincare Solutions
Introduction
Bee Venom, a natural substance derived from honeybee stingers, is rich in bioactive compounds such as enzymes (e.g., hyaluronidase, phospholipase A2), peptides (e.g., melittin, apamin), and biogenic amines . Historically used in traditional medicine, it is now widely recognized for its anti-inflammatory, antibacterial, and regenerative properties, making it a versatile ingredient in both therapeutic and skincare applications .
Key Components & Mechanisms
- Melittin (50% of venom):
- Anti-inflammatory: Inhibits inflammatory pathways, reducing pain in conditions like arthritis and sciatica .
- Antibacterial: Effective against acne-causing bacteria and certain antibiotic-resistant strains .

- Collagen Stimulation: Blocks collagen-degrading enzymes, promoting skin elasticity and reducing wrinkles .
- Phospholipase A2 (PLA2) (12% of venom):
- Enhances blood circulation and immune modulation, beneficial for rheumatic diseases and thrombophlebitis .
- Apamin & Adolapin:
- Provide analgesic effects and modulate neural pathways, aiding in chronic pain management .
Therapeutic Applications
- Musculoskeletal Conditions:
- Arthritis & Rheumatism: Clinical studies show significant pain reduction when applied topically or via controlled injections .
- Sciatica & Neuralgia: Combined with oral vitamin therapy, it improves mobility and reduces inflammation .
- Skin Health:
- Acne Treatment: Melittin’s antibacterial properties combat acne lesions .
- Anti-Aging: Stimulates collagen and elastin production, tightening skin and reducing wrinkles (e.g., Renove Vee Tox Bee Venom Mask) .
- Dermatitis & Wound Healing: Shown to alleviate atopic dermatitis and accelerate wound repair .
- Vascular & Immune Support:
- Improves blood flow in thrombophlebitis and exhibits immune-modulating effects in autoimmune conditions .
Usage Guidelines
- Topical Application: Ointments, creams, or gels (e.g., Bee Venom Professional Gel) are safe for daily use, ideal for joint pain or skincare .
- Controlled Injections: Administered by licensed practitioners in cycles (4–5 days followed by a 2–3 day break) to minimize adverse effects .
- Skincare Regimens: Products like Rodial Bee Venom Moisturiser combine venom with hyaluronic acid for enhanced hydration and anti-aging benefits .
Safety & Precautions
- Side Effects: Temporary redness, swelling, or itching may occur. Severe allergic reactions (anaphylaxis) are rare but possible .
- Professional Supervision: Always consult a healthcare provider before using injectable forms or if you have a bee allergy .
- Regulatory Note: Bee venom products are not FDA-approved and should not replace conventional medical treatments .
Why Choose Our Bee Venom Products?
- Purity: Sourced ethically via electric stimulation for minimal harm to bees .
- Scientific Backing: Supported by peer-reviewed research on its antibacterial, anti-aging, and anti-inflammatory efficacy .
- Versatility: Available in creams, serums, patches, and gels tailored for therapeutic or cosmetic use .
Discover the power of nature’s pharmacy with our premium Bee Venom formulations—where tradition meets modern science.
Keywords: Bee Venom benefits, natural anti-aging, arthritis relief, collagen stimulation, antibacterial skincare.