Our growth depends around the superior machines, exceptional talents and consistently strengthened technology forces for Neohesperidine Dihydrochalcone, Our business is dedicated to giving customers with significant and secure top quality products at aggressive price, making each and every customer pleased with our services.
Our growth depends around the superior machines, exceptional talents and consistently strengthened technology forces for China Food Additive and Neohesperidine, For many years, now we have adhered to the principle of customer oriented, quality based, excellence pursuing, mutual benefit sharing. We hope, with great sincerity and good will, to have the honor to help with your further market.
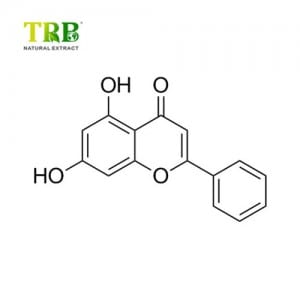
NHDC in pure form is found as a white substance not unlike powdered sugar.
A compound roughly 1500-1800 times sweeter than sugar at threshold concentrations; around 340 times sweeter than sugar weight-for-weight. Its potency is naturally affected by such factors as the application in which it is used, and the pH of the product.
Like other highly sweet glycosides, such as glycyrrhizin and those found in stevia, NHDC’s sweet taste has a slower onset than sugar’s and lingers in the mouth for some time.
Unlike aspartame, NHDC is stable to elevated temperatures and to acidic or basic conditions, and so can be used in applications that require a long shelf life. NHDC itself can stay foodsafe for up to five years when stored in optimal conditions.
The product is well known for having a strong synergistic effect when used in conjunction with other artificial sweeteners such as aspartame, saccharin, acesulfame potassium, and cyclamate, as well as sugar alcohols such as xylitol. NHDC usage boosts the effects of these sweeteners at lower concentrations than would otherwise be required; smaller amounts of other sweeteners are needed. This provides a cost benefit.
Certificate of Analysis
| Product Information | |
| Product Name: | Neohesperidin Dihydrochalcone 98% |
| Other Name: | NHDC |
| Botanical Source: | Bitter Orange |
| Part Used: | Root |
| Batch Number: | TRB-ND-20190702 |
| MFG Date: | July 02,2019 |
|
Item |
Specification | Method | Test Result |
| Active Ingredeints | |||
| Assay(%.On Dried Base) | Neohesperidin DC≧98.0% |
HPLC |
98.19% |
|
Physical Control |
|||
| Appearance | White powder | Organoleptic | Complies |
| Odor& Taste | Characteristic flavor | Organoleptic | Complies |
| Identification | Identical to R.S.samples/TLC | Organoleptic | Complies |
| Particle Size | 100% pass 80mesh | Eur.Ph.<2.9.12> | Complies |
| Loss on Drying | ≦5.0% | Eur.Ph.<2.4.16> | 0.06% |
| Water | ≦5.0% | Eur.Ph.<2.5.12> | 0.32% |
| Bulk Density | 40~60 g/100mL | Eur.Ph.<2.9.34> | 46g/100mL |
| Extract Solvent | Ethanol & Water | / | Complies |
|
Chemical Control |
|||
| Lead(Pb) | ≦3.0mg/kg |
Eur.Ph.<2.2.58>ICP-MS |
Complies |
| Arsenic(As) | ≦2.0mg/kg |
Eur.Ph.<2.2.58>ICP-MS |
Complies |
| Cadmium(Cd) | ≦1.0mg/kg |
Eur.Ph.<2.2.58>ICP-MS |
Complies |
| Mercury(Hg) | ≦0.1mg/kg |
Eur.Ph.<2.2.58>ICP-MS |
Complies |
| Solvent Residual | Meeting USP/Eur.Ph.<5.4> |
Eur.Ph.<2.4.24> |
Complies |
| Pesticides Residual | Meeting USP/Eur.Ph.<2.8.13> |
Eur.Ph.<2.8.13> |
Complies |
|
Microbiological Control |
|||
| Total Plate Count | ≦1,000cfu/g |
Eur.Ph.<2.6.12> |
Complies |
| Yeast & Mold | ≦100cfu/g |
Eur.Ph.<2.6.12> |
Complies |
| E.Coli | Negative |
Eur.Ph.<2.6.13> |
Complies |
| Salmonella sp. | Negative |
Eur.Ph.<2.6.13> |
Complies |
| Packing and Storage | |||
| Packing | Pack in paper-drums. 25Kg/Drum | ||
| Storage | Store in a well-closed container away from moisture and direct sunlight. | ||
| Shelf Life | 2 years if sealed and stored properly. | ||
|
More information of TRB |
||
| Regulation certification | ||
| U.S.FDA,CEP,KOSHER HALAL GMP ISO Certificates | ||
| Reliable Quality | ||
| Nearly 20 years,export 40 countries and regions,more than 2000 batches produced by TRB have no any quality problems,unique purification process,impurity and purity control meet USP,EP and CP | ||
| Comprehensive Quality System | ||
|
|
▲Quality Assurance System |
√ |
| ▲ Document control |
√ |
|
| ▲ Validation System |
√ |
|
| ▲ Training System |
√ |
|
| ▲ Internal Audit Protocol |
√ |
|
| ▲ Suppler Audit System |
√ |
|
| ▲ Equipment Facilities System |
√ |
|
| ▲ Material Control System |
√ |
|
| ▲ Production Control System |
√ |
|
| ▲ Packaging Labeling System |
√ |
|
| ▲ Laboratory Control System |
√ |
|
| ▲ Verification Validation System |
√ |
|
| ▲ Regulatory Affairs System |
√ |
|
| Control Whole Sources and Processes | ||
| Strictly controlled all raw material,accessories and packaging materials.Preferred raw materials and accessories and packaging materials supplier with U.S. DMF number.Several raw material suppliers as supply assurance. | ||
| Strong Cooperative Institutions to support | ||
| Institute of botany/Institution of microbiology/Academy of Science and Technology/University | ||