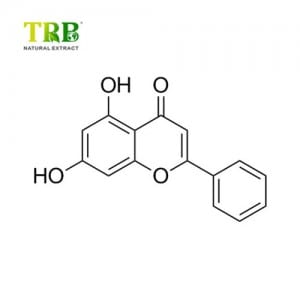
Rutin est compositum e gemma floris germinis extractum (edo glutinosum), quod etiam muscae, Vitaminum P, et sable vocari potest. Vitaminum medicamentum est, quod capillarium permeabilitatem et fragilitatem minuere potest, normalem elasticitatem capillarium conservare et restaurare potest; et ideo adhiberi potest ad impediendum et tractandum hemorrhagia hypertensiva, haemorrhagia diabetica retinae et purpurae hemorrhagic. Eodem tempore potest impedire Vitaminum C ne oxidized, corpus vitaminum C absorbeat, et sanum motus inflammationis promoveat. Insuper, rutin etiam pro cibo antioxidant et illinit.
Product Name: Rutin
Plant Part Used: Semen
Botanical Source:Sophora Japonica Extrac
Intende: ≥80% ab HPLC
Color: luteum ad album pulveris cum ratione odoris et saporis
GMO Status: GMO Free
Stipare: in 25kgs fibra tympana
Repono: Contine continens aediculam in loco frigido, sicco, abstine a luce firma
Shelf Life: 24 months from the date of production
Praecipuum munus:
1. Rutin circulationem sanguinis moderari potest, ne formatio thrombi (sanguinis concreti), resistentia vascularia crescens et fragilitatem vasculi sanguinis et permeabilitatem vascularium reducendo.Usus est tractare haemorrhagia cerebri, haemorrhagia retinae et cetera.
2. Rutin pulveris sanguinem pressuram et sanguinem pinguem reducere potest adiuvat.
3. Rutin extractum habet effectus anti-inflammatorios et anti-allergicos, et in medicamentis ad cutem curandum adhiberi potest.
4. Rutin late in cibo usus est ut antioxidant, muniant agentis vel pigmentum naturale.
Applicatio:
1. Usus est in agro medicinae.
2. In regione sanitatis cura producta ad prohibendos sanguinem morbos, anti-oxidationes et anti-canus medicamenta valetudinis curandas adhibetur.
3. In fucis utendum est ad emulsationes faciendas, mora senescentes cutem tutandam.
Testimonium Analysis
| Product Information | |
| Product nomen: | Rutin |
| Nomen botanicum: | Sophora japonica L. |
| Pars Used: | Flos Sophorae Immaturus |
| Batch numerus: | TRB-SJ-20201228 |
| MFG Date: | Dec 28,2020 |
| Item | Specification | Methodus | Test Proventus |
| Active Ingrediints | |||
| Temptare (% .On arida base) | Rutin≧95.0% | HPLC | 95.15% |
| Corporalis Imperium | |||
| Aspectus | Flavo viridi pulveris | Organoleptic | Obsequitur |
| Odoris & Taste | Saporem proprium | Organoleptic | Obsequitur |
| Lepidium sativum | Identalis RSsamples / TLC | Organoleptic | Obsequitur |
| Particulus Size | C% transiet 80mesh | Eur. Ph.<2.9.12> | Obsequitur |
| Damnum in Siccatio | -5.0% | Eur. Ph.<2.8.17> | 2.30% |
| Summa Cinerum | -10.0% | Eur. Ph.<2.4.16> | 0.06% |
| Mole Density | 40~60 g/100mL | Eur. Ph.<2.9.34> | 49g/100mL |
| Extract Solvent | Ethanol & Aqua | / | Obsequitur |
| Imperium chemica | |||
| Duc (Pb) | 3.0mg/kg | Eur.Ph.<2.2.58>ICP-MS | Obsequitur |
| Arsenicum (As) | ≦2.0mg/kg | Eur.Ph.<2.2.58>ICP-MS | Obsequitur |
| Cadmium (Cd) | 1.0mg/kg | Eur.Ph.<2.2.58>ICP-MS | Obsequitur |
| Mercurius (Hg) | 0.1mg/kg | Eur.Ph.<2.2.58>ICP-MS | Obsequitur |
| Residua solvendo | Occurrens USP/Eur.Ph.<5.4> | Eur. Ph.<2.4.24> | Obsequitur |
| Pesticides Residua | Occurrens USP/Eur.Ph.<2.8.13> | Eur. Ph.<2.8.13> | Obsequitur |
| Imperium Microbiologicum | |||
| Totalis comitis Plate | ≦1,000cfu/g* | Eur. Ph.<2.6.12> | Obsequitur |
| Fermentum & Mold | 100cfu/g | Eur. Ph.<2.6.12> | Obsequitur |
| E.Coli | Negative | Eur. Ph.<2.6.13> | Obsequitur |
| Salmonella sp. | Negative | Eur. Ph.<2.6.13> | Obsequitur |
| Stipare et at | |||
| stipare | Stipant in charta-tympana.25Kg/Drum | ||
| Repono | Condite in vase bene clauso ab umore et a sole directo. | ||
| Fasciae vita | 2 annis signatus et rite repositus. | ||